Spray dried dispersion (SDD) plays a crucial role in pharmaceuticals by improving the bioavailability of drugs. This process enhances the solubility and stability of poorly water-soluble drugs, making them more effective for patients. In this comprehensive guide, we will explore the concept of spray dried dispersion, its applications, the process involved, and the benefits it offers in drug development.
What is Spray Dried Dispersion?
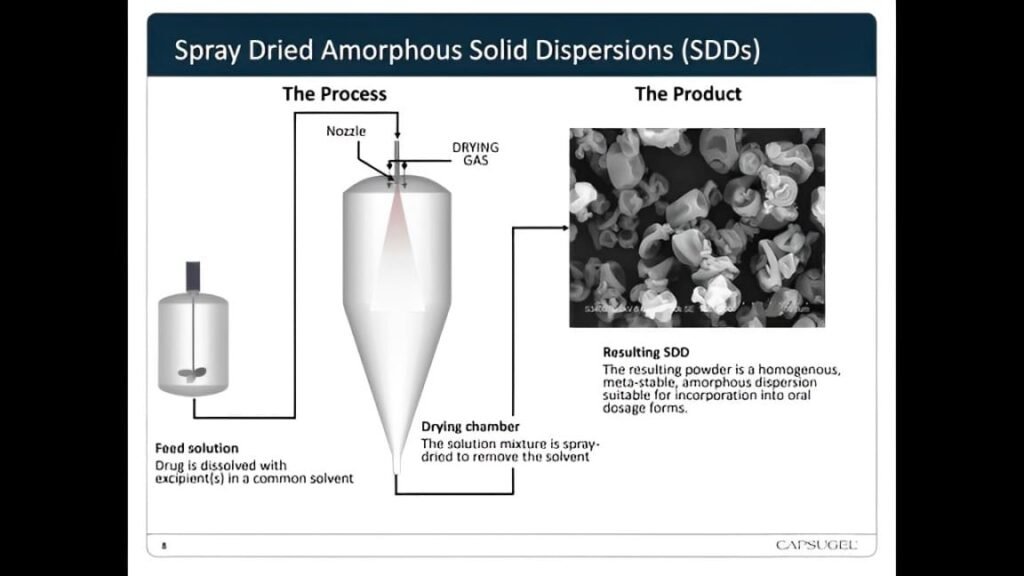
Spray dried dispersion improves the solubility and bioavailability of poorly water-soluble drugs. The process involves dispersing the drug in a polymer matrix and then spray drying the mixture to form a solid dispersion. This technique transforms the drug from a crystalline to an amorphous state, enhancing its dissolution rate and bioavailability.
The Spray Drying Process for Dispersions
The spray drying process for dispersions involves several critical steps:
1. Preparation of the Drug-Polymer Solution: First, you prepare a solution or suspension of the drug and a suitable polymer. The polymer must be compatible with the drug and enhance its solubility. Commonly used polymers include hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone (PVP), and ethyl cellulose.
2. Atomization: Next, you atomize the drug-polymer solution. This step breaks the solution into fine droplets using a spray dryer. The two primary types of atomizers used in this process are rotary atomizers and nozzle atomizers. Rotary atomizers use a high-speed rotating disk to disperse the solution, while nozzle atomizers force the solution through a small orifice to create a fine spray.
3. Drying: After atomization, the fine droplets enter a drying chamber where they contact hot air. The temperature of the hot air ranges from 100°C to 200°C, depending on the properties of the drug and polymer. As the droplets encounter the hot air, the solvent rapidly evaporates, leaving behind solid particles of the drug dispersed in the polymer matrix.
4. Collection: Finally, you collect the solid particles using a cyclone separator or a bag filter. These particles, known as spray dried dispersions, are then characterized for their physical and chemical properties to ensure they meet the desired specifications.
Applications of Spray Dried Dispersion
Spray dried dispersion has numerous applications in the pharmaceutical industry, particularly in the development of oral solid dosage forms. Some key applications include:
1. Improving Bioavailability: Spray dried dispersion significantly improves the bioavailability of poorly water-soluble drugs. By transforming the drug into an amorphous state and dispersing it in a polymer matrix, SDD enhances the drug’s dissolution rate, leading to better absorption in the body.
2. Enhancing Stability: Spray dried dispersion enhances the stability of drugs. The amorphous form of the drug is more stable than its crystalline counterpart, reducing the risk of degradation and improving shelf life.
3. Formulating Controlled Release Drugs: You can use SDD to formulate controlled release drug products. The polymer matrix can release the drug at a controlled rate, ensuring a sustained therapeutic effect over an extended period.
4. Developing Combination Drugs: SDD enables the development of combination drugs by dispersing multiple drugs in a single polymer matrix. This approach simplifies the formulation process and improves patient compliance.
Benefits of Spray Dried Dispersion
Spray dried dispersion offers several benefits in drug development and formulation:
1. Enhanced Solubility and Bioavailability: SDD significantly improves the solubility and bioavailability of poorly water-soluble drugs, making them more effective for patient use.
2. Improved Stability: The amorphous form of the drug in the polymer matrix is more stable than its crystalline form, reducing the risk of degradation and improving shelf life.
3. Versatility: Spray dried dispersion is a versatile technique that can be used to formulate a wide range of drugs, including small molecules, peptides, and biologics.
4. Cost-Effective: SDD is a cost-effective method for enhancing the solubility and bioavailability of drugs. It allows for the development of high-quality drug products without the need for expensive and time-consuming formulation processes.
5. Scalability: The spray drying process is easily scalable, making it suitable for both small-scale and large-scale production. This flexibility ensures that SDD can be used in various stages of drug development, from preclinical studies to commercial manufacturing.

Key Considerations in Spray Dried Dispersion
When developing spray dried dispersions, you must consider several key factors to ensure the success of the formulation:
1. Selection of Polymer: Choose a polymer that is compatible with the drug, enhances its solubility, and provides the desired release profile. Commonly used polymers include hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone (PVP), and ethyl cellulose.
2. Solvent Selection: Select a solvent that can dissolve both the drug and polymer and is easily removed during the spray drying process. Common solvents used in SDD include water, ethanol, and acetone.
3. Optimization of Process Parameters: Optimize the spray drying process parameters, such as inlet temperature, feed rate, and atomization method, to ensure the formation of high-quality spray dried dispersions. These parameters can significantly impact the particle size, morphology, and stability of the final product.
4. Characterization of Spray Dried Dispersions: Thoroughly characterize the spray dried dispersions to ensure they meet the desired specifications. This includes evaluating the particle size distribution, morphology, solid state properties, and dissolution rate of the dispersions.
5. Regulatory Considerations: Consider the regulatory requirements when developing spray dried dispersions for pharmaceutical use. Ensure the safety and efficacy of the formulation and comply with guidelines set forth by regulatory agencies such as the FDA and EMA.
Advances in Spray Dried Dispersion Technology
Recent advances in spray dried dispersion technology have further enhanced its capabilities and applications in drug development:
1. Advanced Atomization Techniques: New atomization techniques, such as ultrasonic atomization, offer improved control over droplet size and distribution. This results in more uniform particle sizes and enhanced product quality.
2. Real-Time Process Monitoring: Advanced process monitoring technologies provide real-time data on the spray drying process, allowing for precise control and optimization of the parameters. This ensures the consistent production of high-quality spray dried dispersions.
3. Integration with Continuous Manufacturing: Integrating spray dried dispersion with continuous manufacturing processes offers significant advantages in terms of efficiency and scalability. Continuous manufacturing allows for the production of large quantities of spray dried dispersions in a streamlined and cost-effective manner.
4. Use of Novel Polymers: The development of novel polymers with enhanced solubilizing properties has expanded the applications of spray dried dispersion. These new polymers offer improved compatibility with a wider range of drugs, further enhancing the solubility and bioavailability of challenging compounds.
5. Formulation of Biologics: Spray dried dispersion technology is now being applied to the formulation of biologics, including peptides and proteins. This offers new opportunities for improving the stability and delivery of these complex molecules.
Conclusion
Spray dried dispersion is a powerful technique in pharmaceutical development, offering significant benefits in terms of solubility, bioavailability, and stability. By understanding the process and key considerations involved, pharmaceutical professionals can leverage this technology to develop high-quality drug products that meet the needs of patients. Advances in spray dried dispersion technology continue to expand its applications and capabilities, making it an essential tool in modern drug development.
For more in-depth articles and resources on pharmaceutical technologies and innovations, visit EngiTech.in. Stay updated on the latest advancements and applications in the industry!



