Spray Drying Pharmaceuticals: Process & Benefits
Spray drying plays a crucial role in the pharmaceutical industry by improving the solubility, stability, and bioavailability of pharmaceutical compounds. This detailed guide explores the spray drying process, its applications in pharmaceuticals, the benefits it offers, and the latest advancements in this field.
What is Spray Drying?
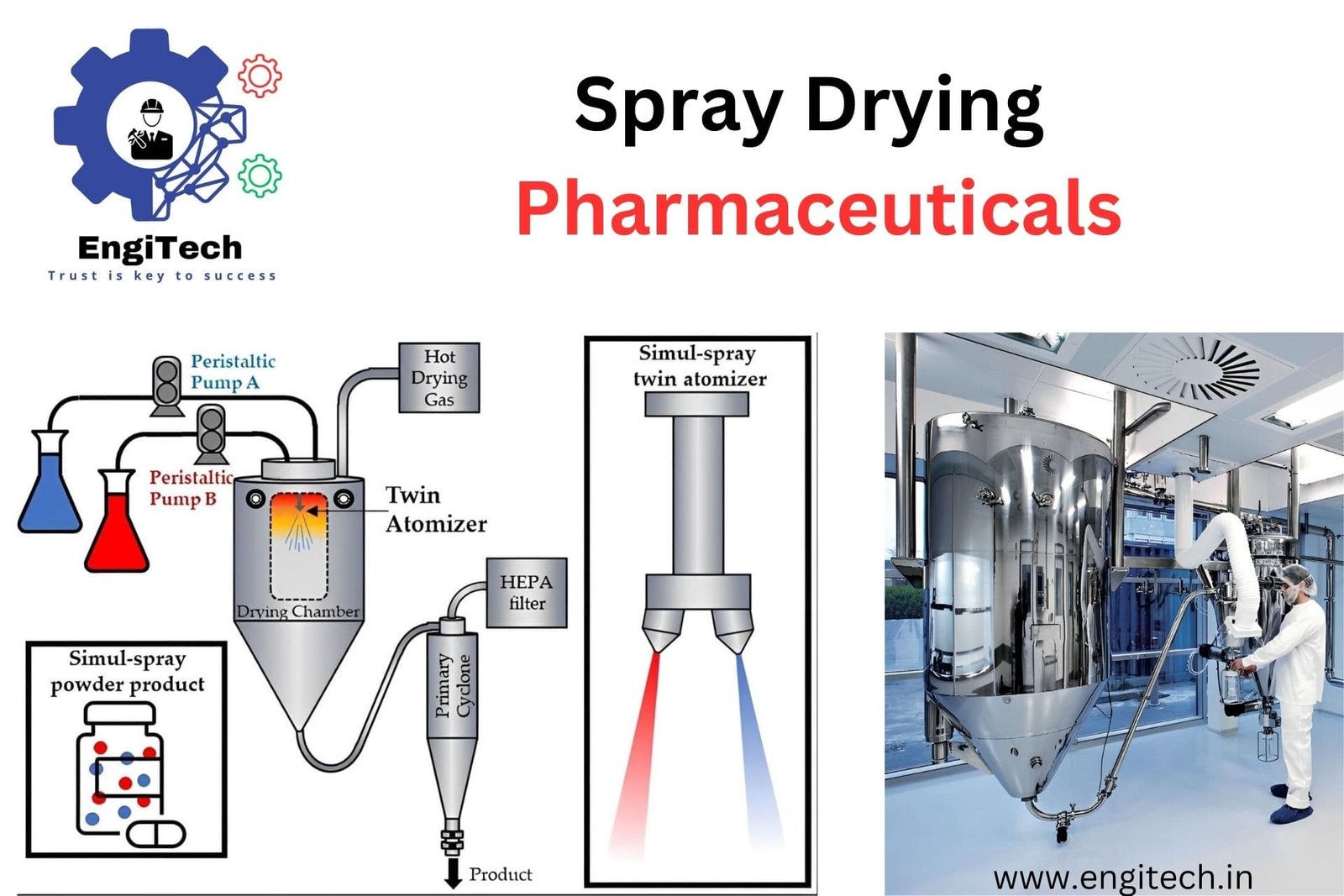
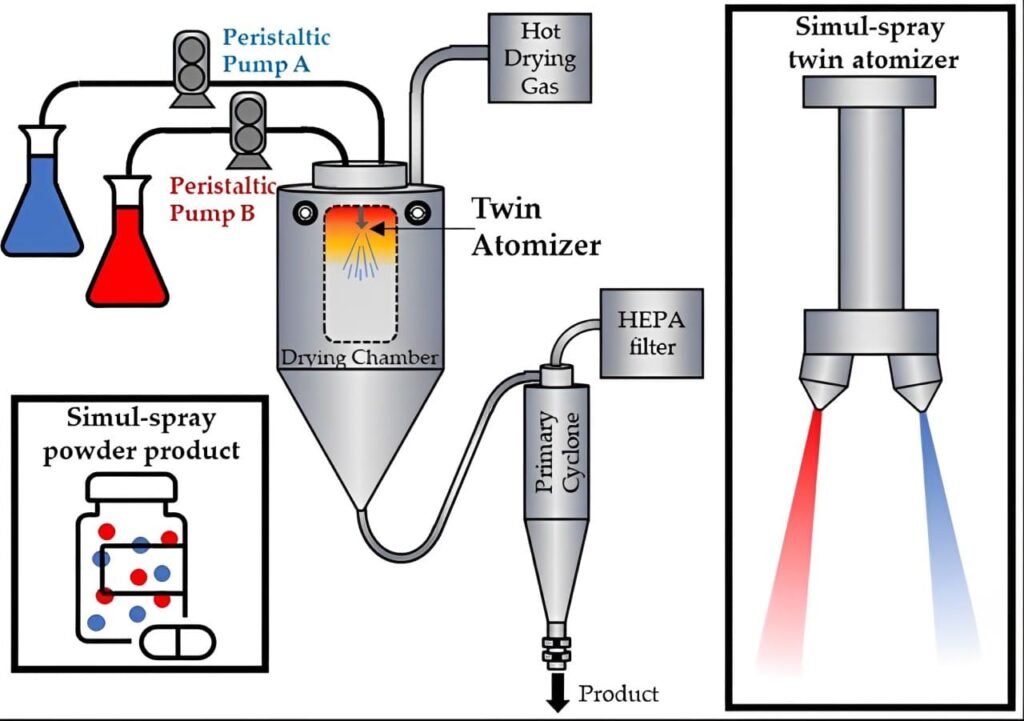
Spray drying converts a liquid or slurry into a dry powder by rapidly drying it with a hot gas. In pharmaceuticals, the primary goal of spray drying is to produce a fine, consistent, and stable powder for various drug formulations. The process involves three main steps: atomization, drying, and collection.
The Spray Drying Process in Pharmaceuticals
1. Atomization: In the first step, you atomize the liquid feed (a solution or suspension of the pharmaceutical compound) into fine droplets using a spray dryer. The atomizer, either a rotary atomizer or a nozzle atomizer, breaks the liquid into tiny droplets, increasing the surface area for efficient drying.
2. Drying: Next, the fine droplets enter a drying chamber where they contact hot air. The hot air temperature ranges from 100°C to 200°C, depending on the thermal sensitivity of the pharmaceutical compound. As the droplets encounter the hot air, the solvent rapidly evaporates, leaving behind solid particles.
3. Collection: Finally, you collect the solid particles using a cyclone separator or a bag filter. These particles, now in the form of a dry powder, are then further processed or formulated into the final pharmaceutical product.

Spray Drying Pharmaceuticals

Applications of Spray Drying in Pharmaceuticals
Spray drying offers a wide range of applications in the pharmaceutical industry:
1. Enhancing Solubility and Bioavailability: By converting drugs into an amorphous state and dispersing them in a polymer matrix, spray drying improves the solubility and bioavailability of poorly water-soluble drugs.
2. Producing Inhalable Powders: Spray drying produces inhalable powders for pulmonary drug delivery. The fine, uniform particles are ideal for delivery to the lungs, where they can be rapidly absorbed into the bloodstream.
3. Formulating Controlled Release Drugs: You can use spray drying to formulate controlled release drug products. The polymer matrix can release the drug at a controlled rate, providing a sustained therapeutic effect over an extended period.
4. Developing Vaccines and Biologics: Spray drying stabilizes sensitive biological molecules and converts them into a dry powder form, making vaccines and biologics easier to store and transport.
5. Encapsulating Active Pharmaceutical Ingredients (APIs): Spray drying encapsulates APIs in a protective matrix, shielding them from environmental factors such as moisture, heat, and light. This encapsulation enhances the stability and shelf life of the drug.
Benefits of Spray Drying in Pharmaceuticals
Spray drying offers several benefits in pharmaceutical development and manufacturing:
1. Enhanced Solubility and Bioavailability: Spray drying significantly improves the solubility and bioavailability of poorly water-soluble drugs, making them more effective for patient use.
2. Improved Stability: The amorphous form of the drug produced by spray drying is more stable than its crystalline counterpart, reducing the risk of degradation and improving shelf life.
3. Uniform Particle Size: Spray drying produces particles with a uniform size and shape, essential for consistent drug formulation and delivery.
4. Scalability: You can easily scale the spray drying process, making it suitable for both small-scale and large-scale production. This flexibility ensures that spray drying can be used in various stages of drug development, from preclinical studies to commercial manufacturing.
5. Cost-Effective: Spray drying is a cost-effective method for producing high-quality pharmaceutical powders. It allows for efficient use of materials and energy, reducing overall production costs.
Key Considerations in Spray Drying Pharmaceuticals
When implementing spray drying in pharmaceutical development, consider these key factors:
1. Selection of Solvent: Choose a solvent that can dissolve the pharmaceutical compound and be easily removed during the drying process. Common solvents used in spray drying include water, ethanol, and acetone.
2. Optimization of Process Parameters: Optimize the spray drying process parameters, such as inlet temperature, feed rate, and atomization method, to ensure the formation of high-quality spray dried powders. These parameters can significantly impact the particle size, morphology, and stability of the final product.
3. Selection of Polymer: Select a polymer compatible with the drug and enhance its solubility. Commonly used polymers include hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone (PVP), and ethyl cellulose.
4. Characterization of Spray Dried Powders: Thoroughly characterize the spray dried powders to ensure they meet the desired specifications. Evaluate the particle size distribution, morphology, solid-state properties, and dissolution rate of the powders.
5. Regulatory Considerations: Consider the regulatory requirements when developing spray dried pharmaceuticals. Ensure the safety and efficacy of the formulation and comply with guidelines set forth by regulatory agencies such as the FDA and EMA.
Advances in Spray Drying Technology for Pharmaceuticals
Recent advances in spray drying technology have further enhanced its capabilities and applications in pharmaceutical development:
1. Advanced Atomization Techniques: New atomization techniques, such as ultrasonic atomization, offer improved control over droplet size and distribution, resulting in more uniform particle sizes and enhanced product quality.
2. Real-Time Process Monitoring: Advanced process monitoring technologies provide real-time data on the spray drying process, allowing for precise control and optimization of the parameters. This ensures the consistent production of high-quality spray dried powders.
3. Integration with Continuous Manufacturing: Integrating spray drying with continuous manufacturing processes offers significant advantages in terms of efficiency and scalability. Continuous manufacturing allows for the production of large quantities of spray dried powders in a streamlined and cost-effective manner.
4. Use of Novel Excipients: The development of novel excipients with enhanced solubilizing properties has expanded the applications of spray drying. These new excipients offer improved compatibility with a wider range of drugs, further enhancing the solubility and bioavailability of challenging compounds.
5. Formulation of Biologics: Spray drying technology is now being applied to the formulation of biologics, including peptides and proteins. This offers new opportunities for improving the stability and delivery of these complex molecules.
Conclusion
Spray drying plays a powerful role in pharmaceutical development, offering significant benefits in terms of solubility, bioavailability, and stability. By understanding the process and key considerations involved, pharmaceutical professionals can leverage this technology to develop high-quality drug products that meet the needs of patients. Advances in spray drying technology continue to expand its applications and capabilities, making it an essential tool in modern drug development.
For more in-depth articles and resources on pharmaceutical technologies and innovations, visit EngiTech.in. Stay updated on the latest advancements and applications in the industry!